A set of pharmacological mechanisms suggests that the use of statin medications may be stimulating the development of atherosclerosis and chronic heart failure. Japanese and American researchers have documented the mechanisms by which statin medications may be causing coronary artery calcification. They propose that the guidelines regulating the use of statin medications be critically re-evaluated [Okuyama 2015].
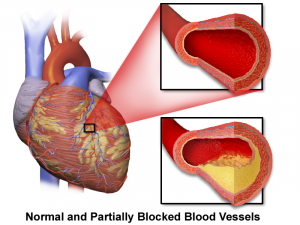
Statin medications reduce total and bad cholesterol levels but may not reduce the extent of atherosclerosis or the risk of chronic heart failure. Attribution: By BruceBlaus [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], from Wikimedia Commons.
First, let’s be clear about our terminology.
Atherosclerosis
Atherosclerosis is the term for damage to the inner walls of the arteries, especially the coronary arteries, leading to hardening and narrowing of those arteries. Hardening of the arteries results in reduced blood flow and reduced transport of oxygen and nutrients to the heart and other tissues and organs.
Chronic heart failure
Chronic heart failure is the term for damage to the heart muscle. The heart muscle becomes weaker and less able to pump adequate quantities of blood, oxygen, and nutrients to the body tissues and organs. Chronic heart failure is a serious condition with a poor prognosis. Adjunctive treatment of chronic heart failure patients with 300 milligrams of Coenzyme Q10 daily improved symptoms and survival [Mortensen 2014].
Statin medications
Statins are medications that are designed to reduce the levels of cholesterol and triglycerides in the blood. Typically, they act by inhibiting the action of the HMG-CoA reductase enzyme that regulates cholesterol production in the body. Unfortunately, inhibiting the action of the HMG-CoA reductase enzyme also inhibits the body’s bio-synthesis of Coenzyme Q10, which is a vital co-factor in the cells’ generation of ATP energy. Supplementation with Coenzyme Q10 is necessary to make up the loss in endogenous Coenzyme Q10.
Problems with statin medications
The Japanese and American researchers discuss the following pharmacological mechanisms by which statin medications increase the risk of atherosclerosis and chronic heart failure.
1. Statins inhibit the bio-synthesis of Coenzyme Q10
The statin medications are toxic to the mitochondria. The mitochondria are the organelles in the cells in which ATP energy generation takes place. By inhibiting the bio-synthesis of both Coenzyme Q10 and heme A, statin medications inhibit the cellular generation of ATP energy molecules. Depriving the heart muscle cells of ATP energy creates an energy-starved heart and causes heart muscle damage and coronary artery damage [Okuyama 2015].
A recent meta-analysis of randomized controlled trials has shown that the four types of statin medications studied — atorvastatin, simvastatin, rosuvastatin, and pravastatin — all cause a significant reduction in plasma Coenzyme Q10 levels. Both lipophilic and hydrophilic statins show a Coenzyme Q10 inhibiting effect [Banach 2015].
2. Statins inhibit the incorporation of selenium into antioxidant selenoproteins
The statin medications inhibit the incorporation of the essential trace element selenium into the antioxidant selenoproteins (= the glutathione peroxidases, the thioredoxin reductases, and the selenoproteins P). Reduced levels of antioxidant selenoproteins results in increased levels of lipid peroxidation, which is associated with increased risk of atherogenesis, carcinogenesis, and ageing [Okuyama 2015].
3. Statins are associated with reduced levels of enzymatic antioxidants
Moreover, the administration of statin medications is known to be associated with reduced levels of the anti-peroxidative enzymes superoxide dismutase and catalase [Okuyama 2015].
Lipid peroxidation is the damage caused by the self-propagating chain reactions of reactive oxygen species reacting with fatty acid membranes whenever enzymatic and non-enzymatic natural antioxidant defenses are overwhelmed. The resulting damage to membrane lipids is dangerous for the survival of cells and tissues.
4. Statins inhibit vitamin K synthesis
Statin medications inhibit the synthesis of vitamin K2, a co-factor that protects arteries against calcification [Okuyama 2015].
Clinical trial results suggesting that statins increase the risk of heart disease
1. Japan Lipid Intervention Trial
When a low dose of simvastatin was used to lower total cholesterol levels below 220 milligrams per deciliter over a six-year period, the result was increased mortality rates for cardiovascular disease, cerebrovascular disease, cancer, and all causes [Okuyama 2015].
Note: Generally, total cholesterol levels between 200 and 239 milligrams per deciliter are considered borderline high and total cholesterol levels above 240 milligrams per deciliter are considered high.
2. US Veterans Statin Follow-up Study
When researchers controlled for such confounding variables as age, baseline characteristics, diabetes, etc., they found that coronary heart disease mortality was higher in the statin-user group than in the non-statin-user group over a five-year period [Okuyama 2015].
3. Danish Cancer Follow-up Study
When researchers adjusted for background cholesterol levels in the statin user group and in the non-user group, they found increased cardiovascular mortality in the statin user group over a 15-year period [Okuyama 2015].
Statin cardiomyopathy and Coenzyme Q10
The Japanese and American researchers use the term “statin cardiomyopathy” to describe impairments in heart muscle function associated with the use of statin medications and not explained by any other pathophysiology. This statin-induced impairment of heart muscle function appears to be common after an average of six years of statin use and appears to be permanent [Okuyama 2015].
- Statins inhibit the bio-synthesis of Coenzyme Q10. The resulting deficient levels of Coenzyme Q10 are associated with increased risk of cardiomyopathy (= chronic disease of the heart muscle) [Folkers 1985].
- Prolonged periods of reduced mitochondrial Coenzyme Q10 levels leads to gradually increased free radical damage to mitochondrial DNA and to progressively reduced ATP energy generation.
- A self-perpetuating vicious cycle results as the mutated mitochondria lose their ability to generate ATP energy and simultaneously produce more harmful free radicals.
Summary: statins and Coenzyme Q10
Patients should be warned about the dangers of long-term statin use:
- Long-term statin use inhibits vitamin K synthesis and enhances thereby calcification of arteries.
- Long-term statin use inhibits bio-synthesis of Coenzyme Q10 and heme A and antioxidant selenoproteins and thereby impairs mitochondrial ATP generation.
- Long-term statin use reduces total cholesterol and bad cholesterol concentrations but increases the risk of coronary heart disease and chronic heart failure.
- Long-term statin use may be implicated in statin-induced adult-onset diabetes mellitus.
Sources
Banach, M., Serban, C., Ursoniu, S., Rysz, J., Muntner, P., Toth, P. P., & … Sahebkar, A. (2015). Statin therapy and plasma coenzyme Q10 concentrations–A systematic review and meta-analysis of placebo-controlled trials. Pharmacological Research, 99329-336. doi:10.1016/j.phrs.2015.07.008
Folkers, K., Vadhanavikit, S., & Mortensen, S. A. (1985). Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proceedings of The National Academy of Sciences of The United States of America, 82(3), 901-904.
Okuyama, H., Langsjoen, P. H., Hamazaki, T., Ogushi, Y., Hama, R., Kobayashi, T., & Uchino, H. (2015). Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Review of Clinical Pharmacology, 8(2), 189-199.
Disclaimer: The information presented in this review article is not intended as medical advice and should not be construed as such.







Leave A Comment