
Dr. Judy has watched the progression of Coenzyme Q10 supplements from dry powder tablets to dry powder in two-piece hard-gel capsules to dry powder in soybean oil in soft-gel capsules to Coenzyme Q10 dissolved in multiple lipids in soft-gel capsules. Today there are even Coenzyme Q10 gummy bears on the market. But what about the formulation? It is the supplement manufacturer’s formulation of the Coenzyme Q10 raw material that is most important for the absorption and health effects.
In line with other technologies, the production technology for Coenzyme Q10 nutritional supplements has advanced over the years. For the past 35 years, Dr. William Judy of the SIBR Research Institute has followed the development of the Coenzyme Q10 nutritional supplements produced in the USA and around the world.
Dr. Judy has seen considerable improvement in the formulations of the Coenzyme Q10 nutritional supplements and in their effectiveness as an adjuvant treatment of chronic heart failure and other clinical conditions. He has seen increasing awareness of the need for Coenzyme Q10 supplements in patients taking statin medications. He has seen increasing awareness of the health benefits for healthy middle-aged and elderly people.
Early research with Coenzyme Q10 supplements
In the beginning, in the very early 1980s, Dr. Judy and his colleagues had only 30-milligram Coenzyme Q10 dry-powder tablets from Japan to work with. Then, somewhat later in the early 1980s, they began to do research with two-piece hard-gel capsules containing dry-powder Coenzyme Q10 and a cellulose filler. These tablets and capsules were taken with a glass of water.
The dry-powder Coenzyme Q10 tablets were perhaps the equivalent of Ford Model T automobile. The first hard-gel Coenzyme Q10 capsules were perhaps the equivalent of the Ford Model A automobile.
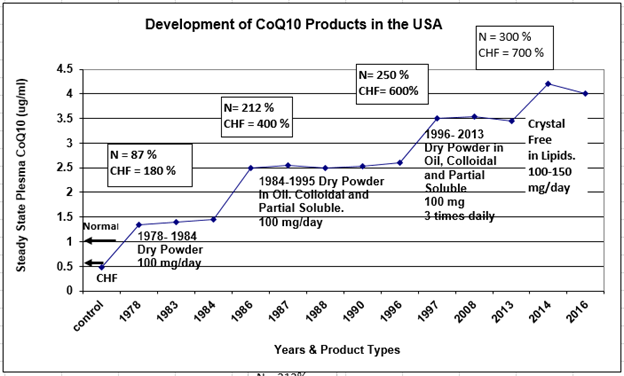
Figure 1: The development of Coenzyme Q10 nutritional supplements in the USA from 1978 to 2016, with the y-values showing the average steady-state plasma bioavailability produced by these supplements in normal adults and in chronic heart failure patients. Note the relative increases from baseline in plasma bioavailability for normal healthy volunteers (N) and for chronic heart failure patients (CHF) with each new product type development. (Attribution: Dr. William V. Judy)
Coenzyme Q10 nutritional supplements in the early 1980’s
The absorption of the early dry-powder tablets and capsules was quite low: equal to or less than 0.7 % of a typical 30-milligram dose of Coenzyme Q10. Long-term supplementation with a daily dose of 100 milligrams of Coenzyme Q10 in these early formulations raised the steady-state plasma bioavailability only to the level of about 1.5 micrograms of Coenzyme Q10 per milliliter of plasma.
Despite the low absorption rate, chronic heart failure patients around the world seemed to respond to these dry powder formulations with increased energy and increased quality of life as compared to placebo formulations. However, the response time until the patients began to show improved cardiac function tended to be quite long, taking months and months of supplementation [Judy].
Supplementation with these early formulations led to relative increases in plasma Coenzyme Q10 concentrations from baseline levels: relative increases of about 87% in normal volunteers and about 200 % in Class III and Class IV chronic heart failure patients (see Figure 1).
The percentage increase was higher in chronic heart failure patients because, being ill, the patients had lower baseline plasma Coenzyme Q10 concentrations.
Coenzyme Q10 nutritional supplementation, 1983 – 1995
In 1983, Dr. Karl Folkers, director of the Institute for Biomedical Research in Austin, Texas, began to provide researchers with the first soft-gel Coenzyme Q10 capsules to be used in clinical research. These capsules contained dry-powder Coenzyme Q10 in soybean oil.
Soft-gel Coenzyme Q10 capsules
The soft-gel capsules, still with dry-powder Coenzyme Q10 in soybean oil inside, were the most advanced form of Coenzyme Q10 nutritional supplement in the USA in the late 1980s and early 1990s. The advance to the soft-gel capsules was perhaps equivalent to Ford’s developing the Thunderbird with a V-8 engine.
Coenzyme Q10 nutritional supplements, 1996 – 2006
Between 1996 and 2006, the advance in Coenzyme Q10 supplement formulation involved the encapsulation of the dry-powder Coenzyme Q10 with one or more oils. Some oils helped absorption more than others.
With the new generation of supplements, the absorption rate increased to 2.4 % to 2.5 % of a 100-milligram dose. The steady-state bioavailability in normal and chronic heart failure patients increased to levels between 2.4 and 2.6 micrograms per milliliter. Chronic heart failure patients began to respond more rapidly to adjuvant treatment with the nutritional supplements that gave this higher plasma Coenzyme Q10 concentration.

The formulation of Coenzyme Q10 nutritional supplements has advanced nearly as much as the production of automobiles has. And it is the formulation of the Coenzyme Q10 nutritional supplement that is decisive for good absorption and bioavailability and, consequently, for beneficial heart health effects. It is important to choose a Coenzyme Q10 supplement with documented research effects.
Encapsulating the dry-powder Coenzyme Q10 with a lipid filler (one or more oils) was perhaps equivalent to Ford’s development of the Mustang.
Desirable plasma Coenzyme Q10 status
The International Coenzyme Q10 Association recommended that adult individuals’ plasma Coenzyme Q10 concentrations be raised to and maintained at the level of 2.5 micrograms per milliliter for optimal heart health.
The newer lipid-based products were used by investigators over a span of more than 10 years. During this period, 1996-2006, there were again considerable relative increases in plasma Coenzyme Q10 levels in normal healthy individuals and in chronic heart failure patients treated with 100 milligrams of Coenzyme Q10 daily:
- normal healthy individuals 212 %
- chronic heart failure patients 400 %
Improved absorption with divided daily doses
In 2005, Singh and a team of researchers reported the following research results concerning the absorption of Coenzyme Q10.
- The researchers found a dose-dependent response to Coenzyme Q10 supplementation that was not necessarily linear. Supplementation with 200 milligrams gave a greater volume of absorption but not twice as great.
- Preheating and dissolving the Coenzyme Q10 raw material in an oil matrix resulted in a supplement that gave better absorption than the older formulations did.
- Dividing the 200-milligram dosages into 100 milligrams two times daily gave better absorption than one single daily 200-milligram did [Singh 2005].
Increased plasma Coenzyme Q10 bioavailability
With the new formulation and with the divided dosage of 100 milligrams twice daily, researchers began to think in terms of raising the plasma bioavailability level of chronic heart failure patients to the level of 3.0 to 3.5 micrograms per milliliter.
Coenzyme Q10 researchers began to give higher daily doses split into two or three times per day. This mode of dosing was used worldwide. The new formulations and dosages led to relative increases in plasma bioavailability concentrations from baseline values of 337 % in normal healthy individuals and 600 % in chronic heart failure patients.
Again, the increase in the chronic heart failure patients is greater because of the lower baseline starting point.
Coenzyme Q10 supplementation 2006 – 2016
In the period 2006 through 2016, the crystal-free multi-lipid Coenzyme Q10 product in various lipid formulations entered the USA market. These were formulations that successfully dissociated the dry-powder raw material Coenzyme Q10 crystals to single Coenzyme Q10 molecules. Some of these new products were unstable; the Coenzyme Q10 re-crystallized when the gelatin capsules were cooled to room temperature. It is a tricky process.
Remember: The body cannot absorb a crystal of any substance.
Much higher Coenzyme Q10 absorption with the new formulations
Testing of the newer Coenzyme Q10 capsules that had little or no re-crystallization inside the capsule showed that the single-dose absorption rate could be increased to between 6.8 % and 8.0 % of a 100-milligram dose. Testing of the Coenzyme Q10 capsules with considerable re-crystallization inside the capsules, however, showed single-dose absorption rates in the range of 2.4 % to 2.6 %.
Higher plasma Coenzyme Q10 bioavailability
The steady-state plasma Coenzyme Q10 bioavailability of these newer formulations with few or no crystals in the capsules tends to be in the range of 3.6 to 4.4 micrograms per milliliter. When, on the other hand, the crystals are present inside the capsules, the steady-state plasma Coenzyme Q10 bioavailability drops to more or less the same level as the level of the older dry-powder crystalline Coenzyme Q10 formulations in a lipid base.
The recent crystal-free Coenzyme Q10 formulations are perhaps equivalent to the Tesla premium electric sedans and SUVs.
Introduction of the ubiquinol supplements to the US market, 2006
In 2006, a new form of Coenzyme Q10 supplement, using the reduced form, known as ubiquinol, entered the US market. Until then, for more than 25 years, the Coenzyme Q10 supplements available for research and for individual use had been exclusively formulations of the ubiquinone form of Coenzyme Q10. Ubiquinone is the stable, much researched oxidized form of the redox molecule Coenzyme Q10.
Ubiquinol is the much less stable, much less researched reduced form of Coenzyme Q10. A single foreign company has a monopoly on the production of the ubiquinol raw material, and ubiquinol is being marketed vigorously without much research documentation to back up the claims for it. I will write more about the development of ubiquinol products in future articles.
Summary
- The ubiquinone Coenzyme Q10 nutritional supplements have advanced in formulation from dry-powder supplements in oil to supplements with the Coenzyme Q10 raw material dissociated into single molecules and encapsulated with carrier lipids.
- The very best Coenzyme Q10 supplement capsules contain few or no crystals at room temperature.
- The ubiquinone Coenzyme Q10 nutritional supplements have been extensively tested in chronic heart failure patients and in healthy elderly individuals. They have shown significant beneficial effects.
- The manufacturer’s formulation of the better ubiquinone Coenzyme Q10 supplements is the decisive difference in the absorption and efficacy of the Coenzyme Q10.
- The ubiquinol supplements are considerably less stable than the ubiquinone Coenzyme Q10 supplements and are very much less researched. The marketing claims for the absorption of ubiquinol supplements are based not on head-to-head comparisons with the best absorbed ubiquinone Coenzyme Q10 supplements but rather on comparisons of results from studies done with different subjects, different study conditions, and different methods of measurement.
Sources:
Folkers, K., Brown, R., Judy, W. V., & Morita, M. (1993). Survival of cancer patients on therapy with Coenzyme Q10. Biochemical And Biophysical Research Communications, 192(1), 241-245.
Judy, W. V. (2017). Private communication.
Judy, W. V., Stogsdill, W. W., & Folkers, K. (1993). Myocardial preservation by therapy with coenzyme Q10 during heart surgery. The Clinical Investigator, 71(8 Suppl), S155-S161








Leave A Comment